Abstract
Allosteric inhibitors of mutant IDH1 or IDH2 induce differentiation of IDH-mutant AML myeloblasts, which in some patients (pts) can lead to a life-threatening differentiation syndrome (DS). The in vivo mechanism of how IDH inhibitors induce differentiation and occasionally DS is not fully understood. Furthermore, responders to the inhibitors often lose the differentiation effect, after median of 7 months. Although a recent study identified secondary mutations in the dimer-interface of IDH1/2 as a mechanism for acquired IDH-inhibitor resistance, given the rarity of this event, it is unlikely to represent the primary mechanism in the majority of pts.
We conducted a comprehensive genomic analysis (DNA sequencing, single-cell DNA sequencing, RNA sequencing and cytosine methylation array) on longitudinally collected bone marrow specimens from IDH1/2-mutant AML pts treated with one of the IDH1/2 inhibitors (ivosidenib, enasidenib, IDH305 or AG881). In total, 146 samples from 39 pts collected at different time points (median 4 [IQR 3-5] samples per patient) were analyzed. 24 pts were IDH1-mutated receiving IDH1 inhibitor and 15 were IDH2-mutated receiving IDH2 inhibitor. 29 pts responded (CR, CRi, CRp or MLFS) and 10 had no response to the inhibitors. 8 of 29 responders developed DS. 22 of 29 responders relapsed after the median of 5 months.
Cytosine methylation analysis revealed significant decrease in global methylation at response than baseline (mean beta value 0.54 at baseline vs. 0.45 at response, P < 0.001). This corroborated with a decrease in serum 2HG level, supporting the role of IDH inhibitors in reversing CpG island methylator phenotype (CIMP) likely through TET restoration. Motif enrichment analysis of differentially methylated promoter regions showed significant de-methylation of PU.1(SPI1) and C/EBP binding motifs at response. Consistent with this, RNA sequencing revealed that PU.1 and C/EBP target genes such as CSF2RA, CSF3R, IL34, TREM2, IL6R, CEBPE, GFI1, and KLF5 had significant upregulation at response. Expression of PU.1 itself was also significantly up-regulated upon response, along with other key hematopoietic differentiation genes and pathways such as GM-CSF, IL8-CXCR1 and IL3 pathways. Pts who developed DS had significantly higher upregulation of PU.1 and GM-CSF pathway compared to other DS-free responders, implicating the potential activation of these pathways in the mechanism of DS. At relapse, these pathways that mediated differentiation were all down-regulated when compared to that at response. Interestingly, methylation level remained suppressed at relapse (mean beta value 0.45 at response vs. 0.47 at relapse, P = 0.566), suggesting that the down-regulation of pro-differentiation signals at relapse is independent of methylation.
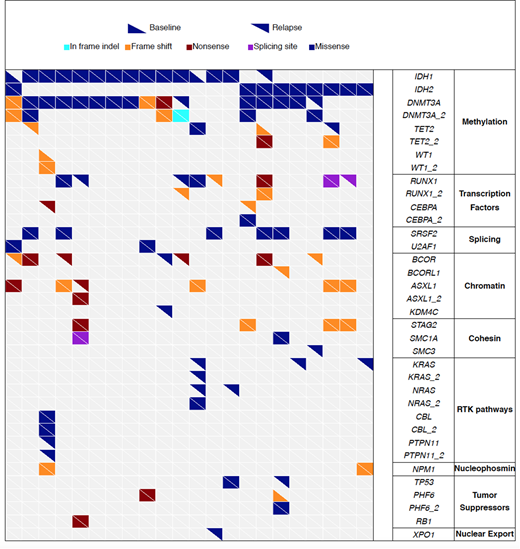
Combined whole exome and targeted deep sequencing revealed the heterogeneous patterns of acquired mutations at relapse. Of 22 cases with baseline and relapse pairs, 18 acquired new driver mutations at relapse (Fig 1). The most frequently acquired mutations at relapse were in BCOR/BCORL1 (N = 6), hematopoietic differentiation genes (CEBPA [N=2] and RUNX1[N=4]), and RTK pathways (NRAS/KRAS [N=5] and PTPN11 [N=1]). We did not identify acquisition of dimer-interface IDH1/2 mutations at relapse. However, in 1 case, relapse was associated with acquisition of mutation in reciprocal IDH gene (IDH2-mutated case acquired IDH1 mutation). Singe-cell DNA sequencing of the double-mutant revealed that the reciprocal acquisition occurred in a different clone.
Analysis of differentially expressed genes between baseline and relapse showed significant upregulation of genes in DNA damage response (DDR) pathways at relapse. Consistent with this, relapse samples showed significantly higher mutation burden than baseline, suggesting that accumulation of mutations are upregulating DDR pathway at relapse.
In summary, IDH inhibitors induce differentiation and DS through upregulation of PU.1 and C/EBP target genes, which is associated with de-methylation of their binding motifs. The mechanisms of acquired resistance to IDH inhibitors are heterogeneous and involves various genes and pathways including BCOR/BCORL1, hematopoietic differentiation transcription factors (CEBPA, RUNX1), and RTK pathways (RAS, PTPN11). In a rare case, acquired mutation in reciprocal IDH gene was detected, occurring in a different clone.
DiNardo:Agios: Consultancy; Abbvie: Honoraria; Medimmune: Honoraria; Bayer: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria. MacBeth:Celgene Corporation: Employment, Equity Ownership. Tosolini:Celgene Corporation: Employment. Frattini:Celgene Corporation: Employment, Equity Ownership. Kadia:Abbvie: Consultancy; Celgene: Research Funding; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Amgen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; BMS: Research Funding; Takeda: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Celgene: Research Funding; Takeda: Consultancy; Abbvie: Consultancy. Ravandi:Xencor: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Bristol-Myers Squibb: Research Funding; Xencor: Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Orsenix: Honoraria; Macrogenix: Honoraria, Research Funding; Seattle Genetics: Research Funding; Orsenix: Honoraria; Jazz: Honoraria; Jazz: Honoraria; Seattle Genetics: Research Funding; Abbvie: Research Funding; Macrogenix: Honoraria, Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria. Konopleva:Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal